Mystery Science respects the intellectual property rights of the owners of visual assets.
We make every effort to use images and videos under appropriate licenses from the owner or by
reaching out to the owner to get explicit permission. If you are the owner of a visual and
believe we are using it without permission, please
contact us—we will reply promptly and make
things right.
Exploration
doll by
Gabby
, used under CC BY
legos by
Priwo
, used under CC BY
superman toy by
JD Hancock
, used under CC BY
toy car by
Emi Yañez
, used under CC BY
old photo by
Grandview This Week
blocks by
Josh Wedin
, used under CC BY
horse by
Garrison Gunter
, used under CC BY-SA
wood car by
Collectie Stichting Nationaal Museum van Wereldculturen
, used under CC BY-SA
carving video by
Gene Messer
, used under CC BY
wooden lion by
Ostheimer Toys
, used under CC BY
xacto knife by
Just plain Bill
zoo pops by
World Wonders
, used under CC BY
ice cubes by
Liz West
, used under CC BY
Ice cube melting by
HeyHondo
, used under CC BY
ice cube tray by
Leif Maxfield
, used under CC BY
yeti ice tray by
Fred & Friends
, used under CC BY
holding ice by
AppleSister
, used under CC BY-SA
wood by
Elke Wetzig
, used under CC BY-SA
Edmund Parkes by
Barraud
, used under CC BY
flask by
Databese Center for Life Science
, used under CC BY
zombie plastic soldiers by
Scientifical Hamster
, used under CC BY
army man by
davidd
, used under CC BY
dinosaur mold by
Ron's Rescued Treasures
, used under CC BY
lego man by
sprout_labs
, used under CC BY
Activity
mailbox by
CGP Grey
, used under CC BY
sign by
Richard Leonard
, used under CC BY
truck driving by
Per
, used under CC BY-SA
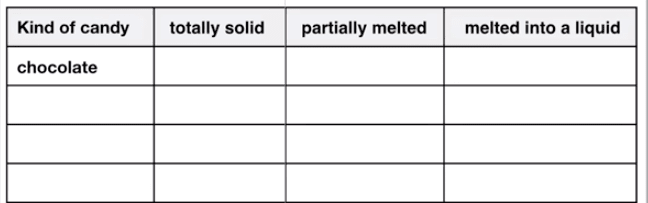
melting chocolate bar by
Shizhao
, used under CC BY-SA
caramel by
Rainer Zenz
, used under CC BY-SA
gummy bears by
David O'Hare
, used under CC BY
starburst candy by
Evan-Amos