Scroll for prep
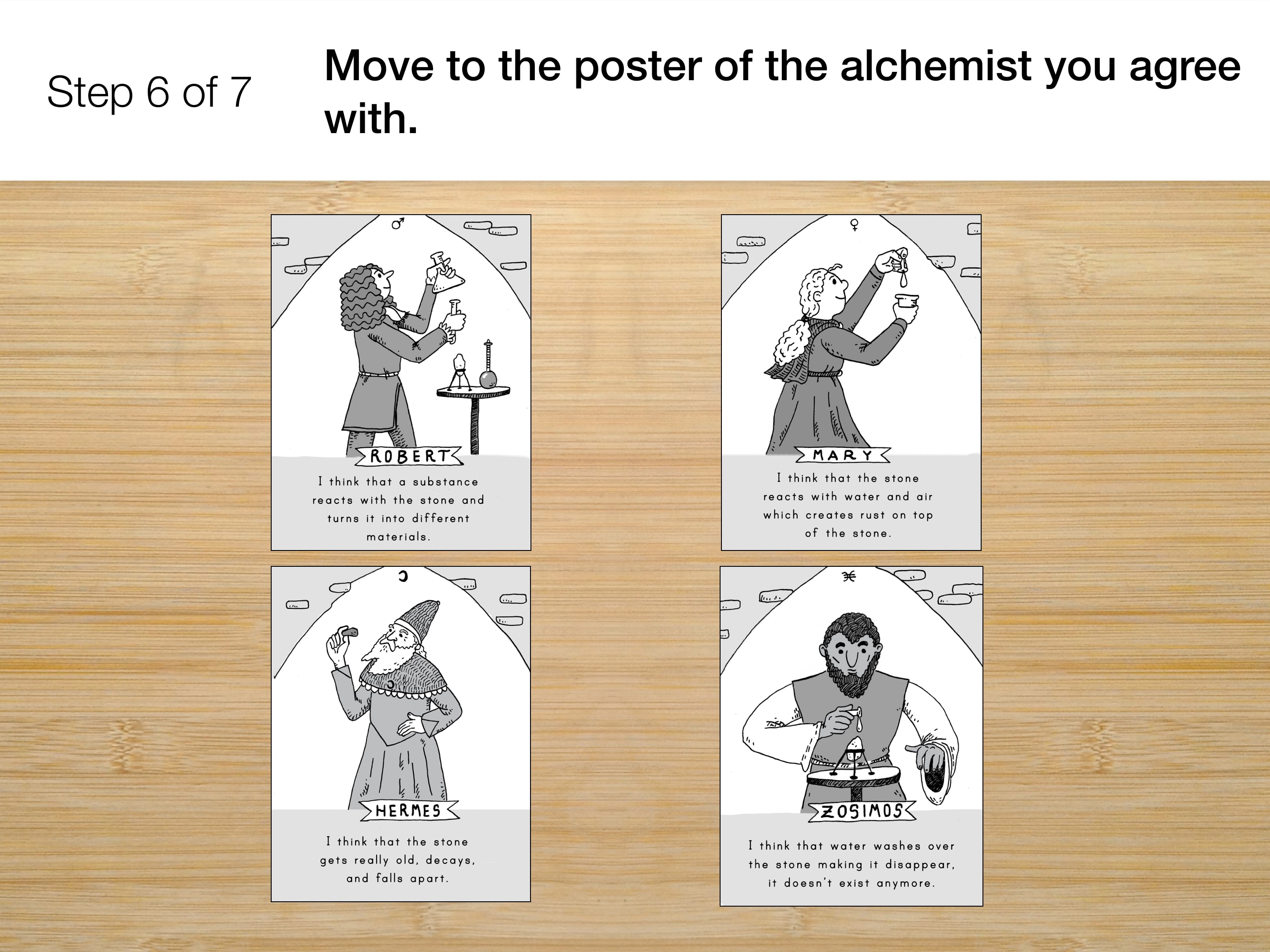
- What caused the gargoyle to change over time?
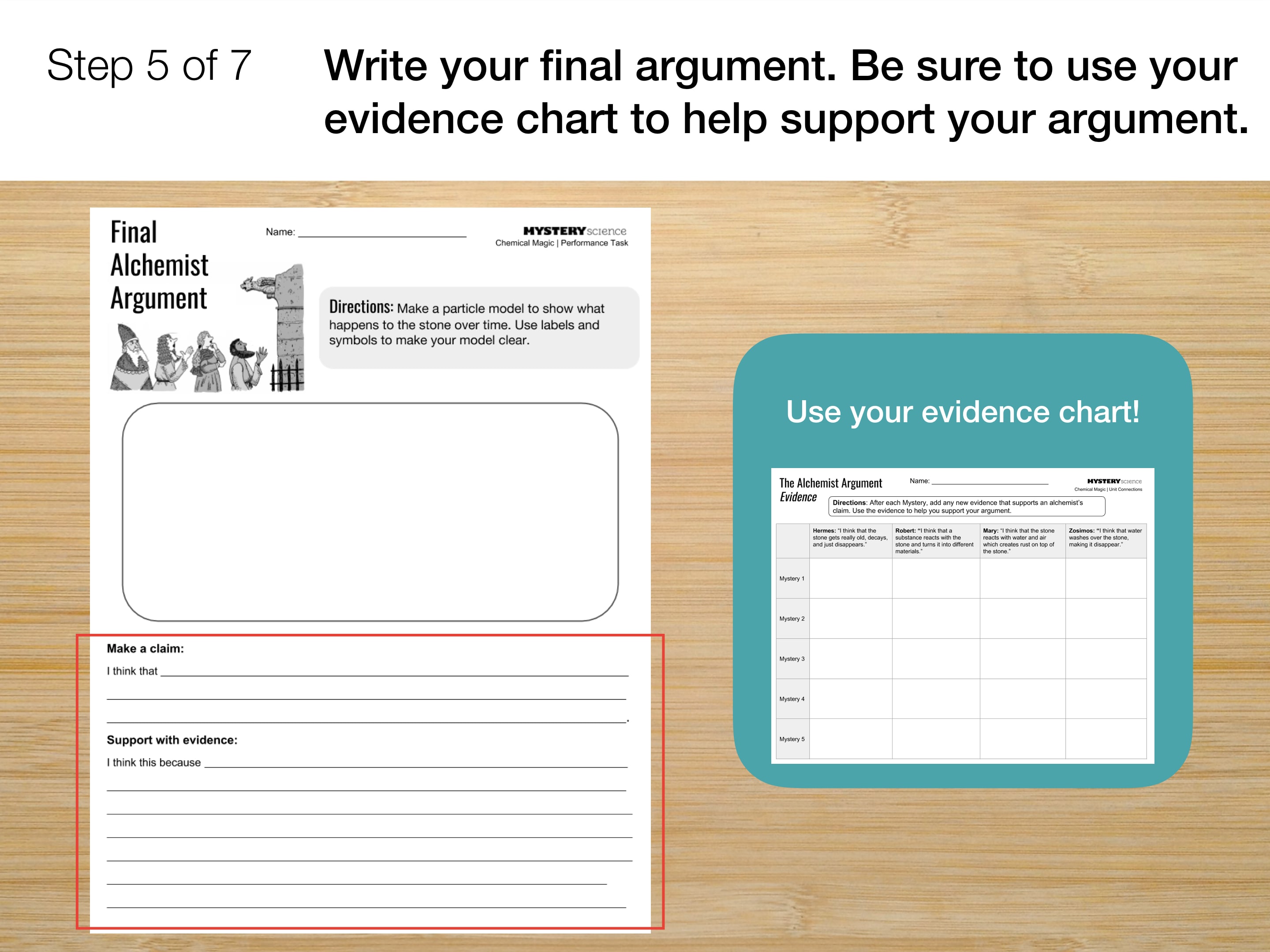
- What pieces of evidence from this unit have helped you explain the change?

- Substances can change other substances
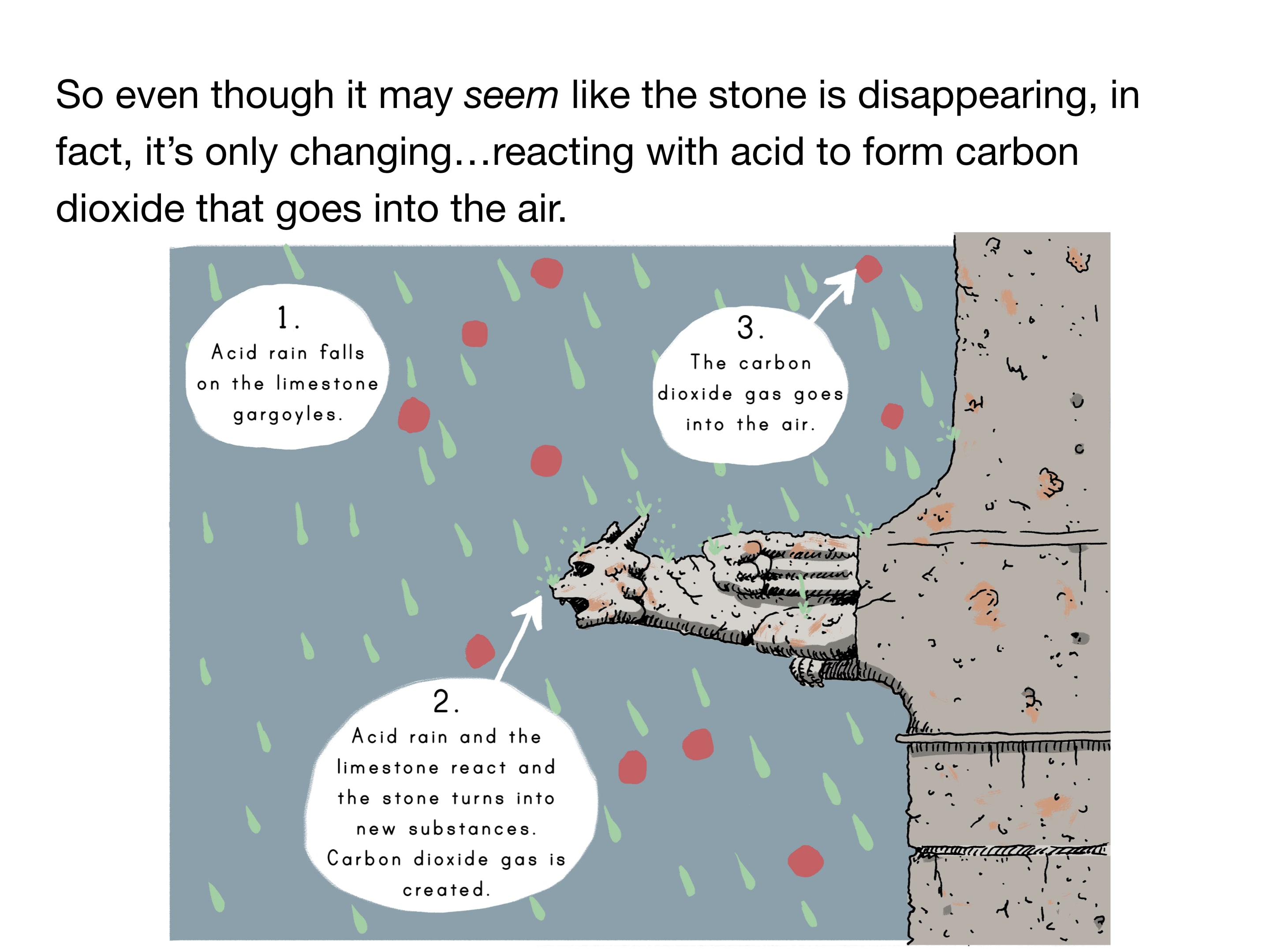
- Substances can change but not disappear
- Acids are very reactive substances
- Chemical reactions are the mixing of two or more substances that create a new substance
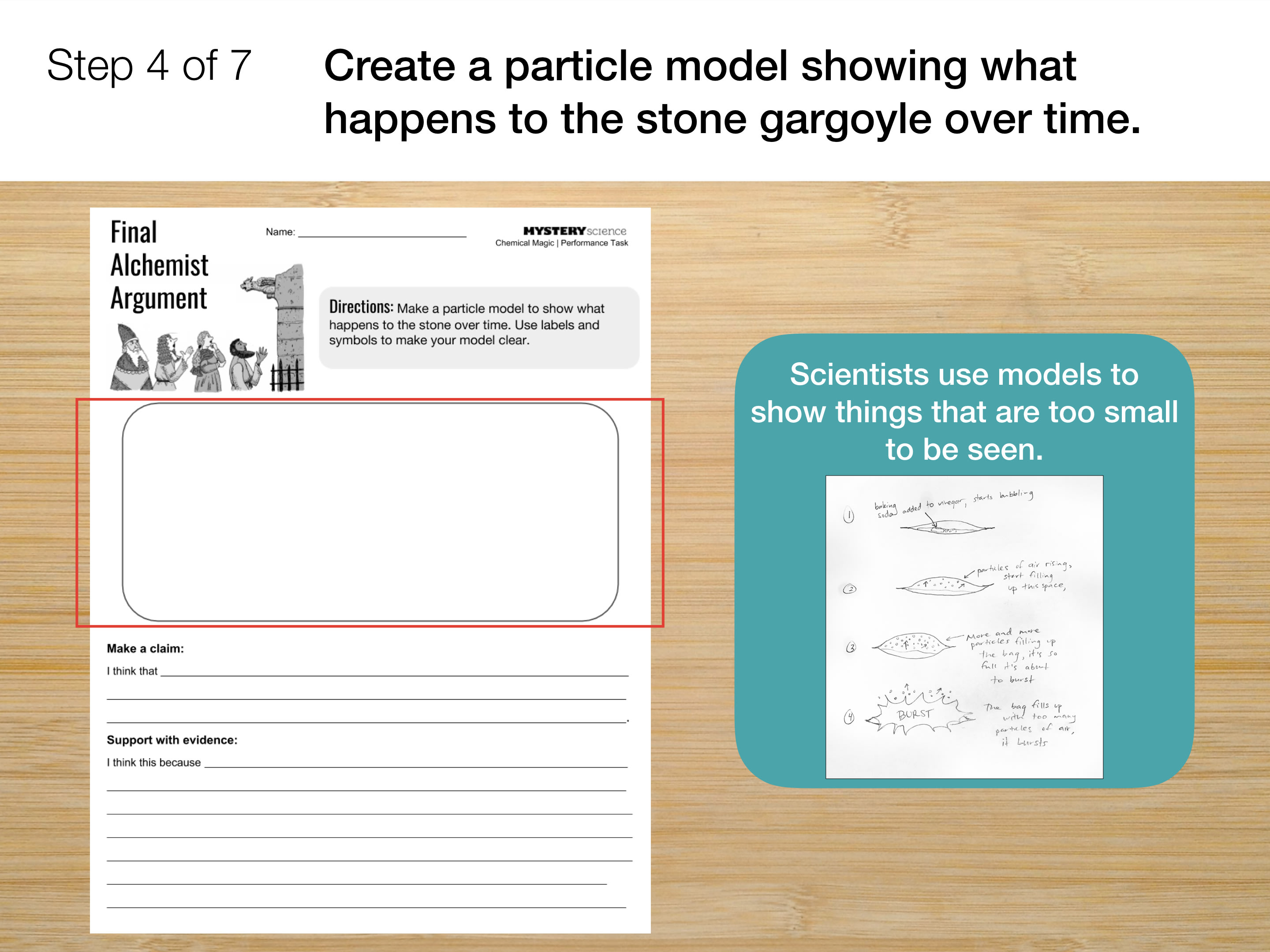
- Gases are made of particles too small to be seen







Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Below are ideas for extending the Performance Task of this unit.
- Activity: Use a chemical reaction to dissolve the shell of an egg.
Activity: Disappearing Eggshell
Follow these instructions, to react vinegar with eggshells and make a naked egg (an egg without a shell)! Eggshells are made of a substance called calcium carbonate, which is the same material the stone gargoyles are made of. The eggshell and vinegar reaction models the reaction between stone gargoyles and acid rain. Try the experiment, and then:
- Draw a particle model that shows what happens to the eggshell in the vinegar.
- Pour the vinegar (with the dissolved shell) onto a flat surface (petri dish, plate). Allow the vinegar to evaporate and see what is left behind!
You've completed the Performance Task!
If you have more time, view the extension activity in the extensions.
This was the last Mystery of Chemical Magic.
View the unit's summative assessment .