Scroll for prep

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
CONVERSEMOS:
Probablemente esta no es la primera vez que has escuchado la palabra "acido." ¿En qué te hace pensar esta palabra?

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
01/19
01/19
Si estás en una clase, encuentra un compañero o compañera
con quien trabajar. Tú y tu compañero o compañera van a trabajar
con otra pareja y compartirán materiales.
con quien trabajar. Tú y tu compañero o compañera van a trabajar
con otra pareja y compartirán materiales.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
02/19
02/19
Cubre tu área de trabajo con un mantel de plástico o con periódico.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
03/19
03/19
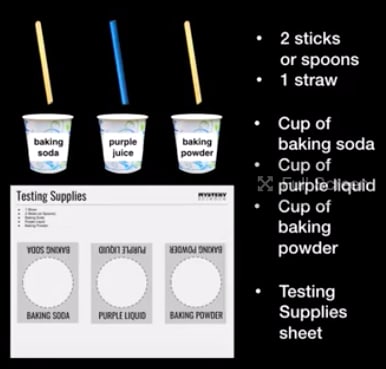
Decidan cuál será el equipo A y cuál será el equipo B. Equipo A:
vayan por los materiales para las reacciones de los ácidos.
Equipo B: vayan por los materiales que pondrán a prueba.
vayan por los materiales para las reacciones de los ácidos.
Equipo B: vayan por los materiales que pondrán a prueba.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
04/19
04/19
Organicen sus materiales en el centro de la mesa. Pongan cada
vaso en el lugar adecuado y luego pongan un popote en cada
líquido y un palito de madera en cada polvo.
vaso en el lugar adecuado y luego pongan un popote en cada
líquido y un palito de madera en cada polvo.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
05/19
05/19
Obtén estos materiales para ti y tu compañero o compañera.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
06/19
06/19
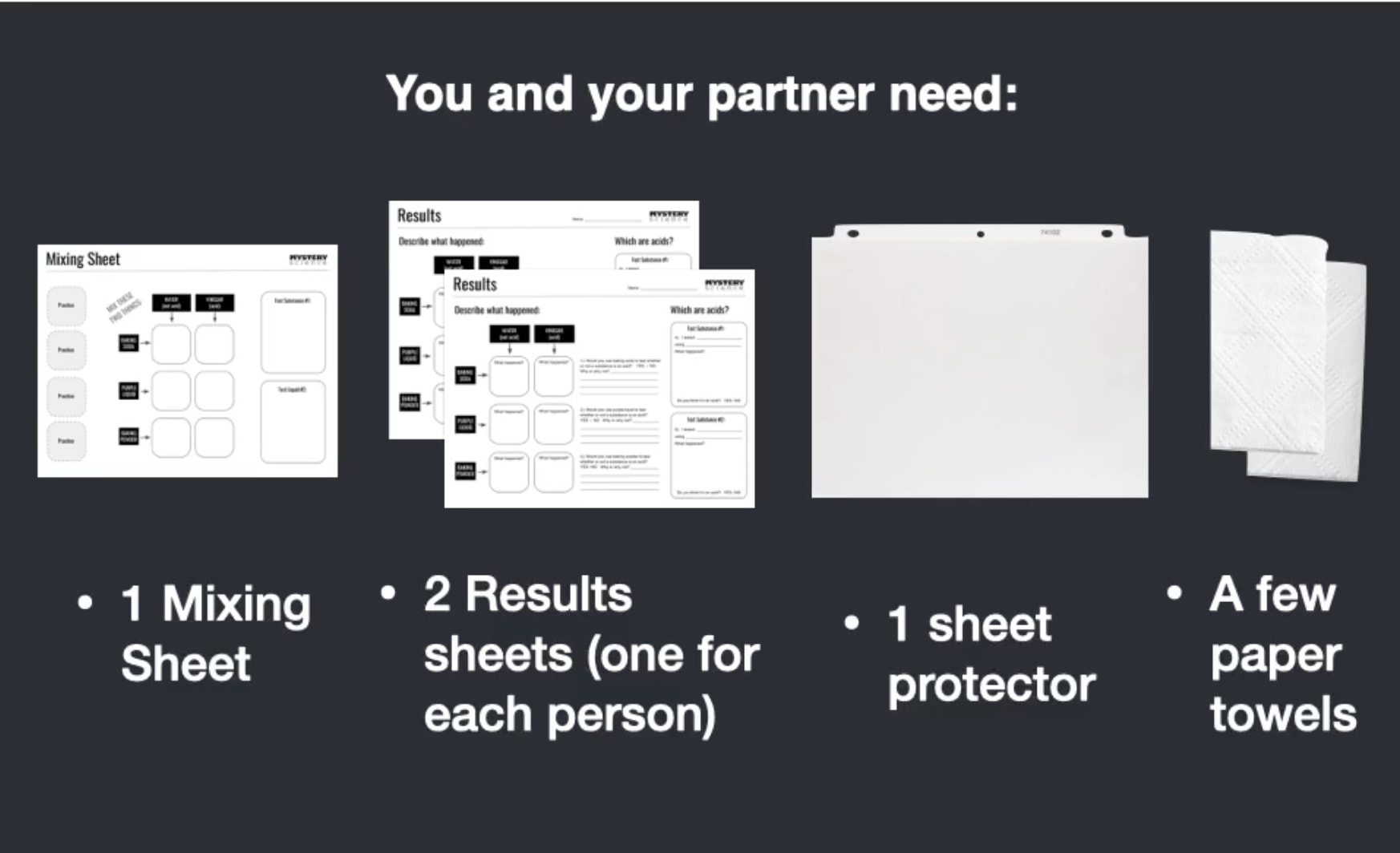
Consigue tu hoja de mezclas y tu protector de hojas. Desliza con
cuidado la hoja de mezclas dentro del protector. Luego asegúrate
que todas tus cosas estén acomodadas así.
cuidado la hoja de mezclas dentro del protector. Luego asegúrate
que todas tus cosas estén acomodadas así.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
07/19
07/19
Practiquemos: Mete el popote en uno de los líquidos. Usa tu dedo
para tapar el popote. Coloca el popote sobre un cuadrito de práctica
y quita tu dedo.
para tapar el popote. Coloca el popote sobre un cuadrito de práctica
y quita tu dedo.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
08/19
08/19
Decide quién será el Cuentagotas y quién será el Probador o
la Probadora.
la Probadora.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
09/19
09/19
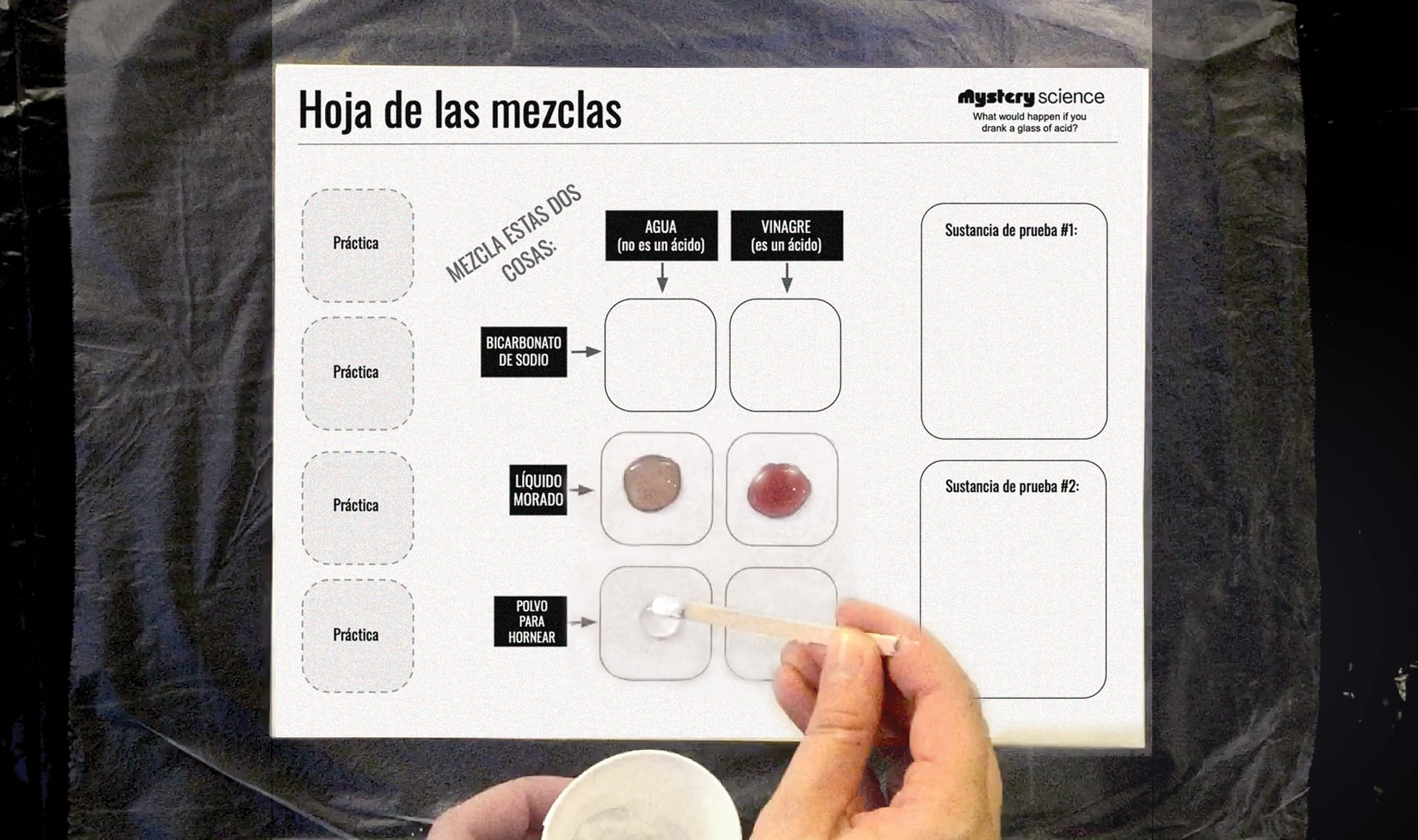
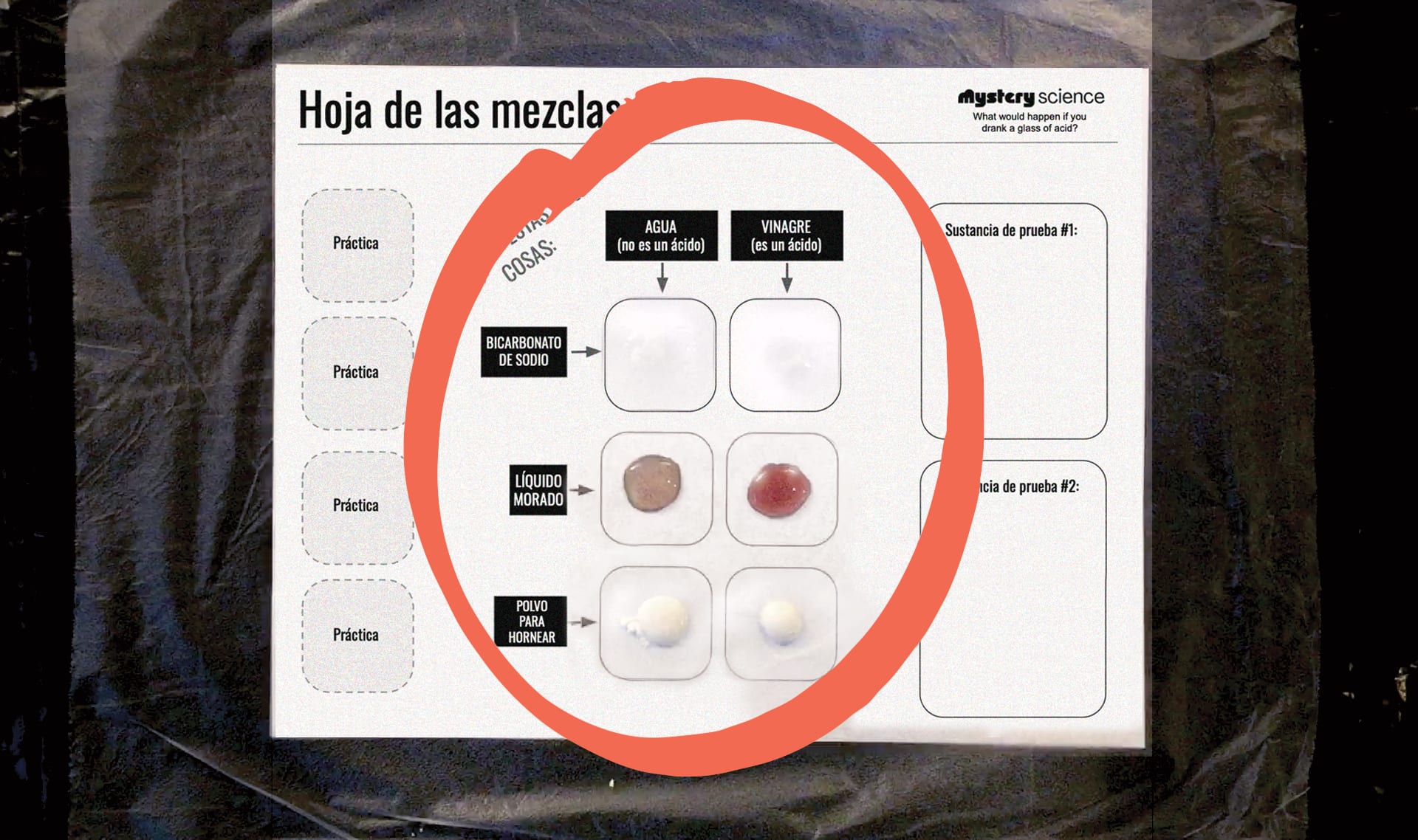
Cuentagotas: pon una gota de agua en el primer cuadrito.
Probador: pon una muestra de bicarbonato de sodio sobre el agua.
Ambos: observen lo que sucede.
Probador: pon una muestra de bicarbonato de sodio sobre el agua.
Ambos: observen lo que sucede.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
10/19
10/19
Ambos: en sus hojas de resultados, describan lo que sucedió cuando
el agua se mezcló con el bicarbonato.
el agua se mezcló con el bicarbonato.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
11/19
11/19
Continua haciendo los experimentos hasta que hayas llenado
todos los cuadritos de prueba. Haz una pausa después de cada
prueba para escribir lo que sucedió en tu hoja de resultados.
todos los cuadritos de prueba. Haz una pausa después de cada
prueba para escribir lo que sucedió en tu hoja de resultados.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
12/19
12/19
Contesta las preguntas número uno, dos y tres.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
13/19
13/19
Platiquen sobre sus respuestas a estas preguntas en equipo.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
14/19
14/19
Obtén una nueva sustancia, un palillo de dientes y un popote si
es un líquido, o un palillo si es un polvo.
es un líquido, o un palillo si es un polvo.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
15/19
15/19
Pon una gota de tu sustancia en este cuadrito, en tu hoja de
mezclas. Haz la prueba.
mezclas. Haz la prueba.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
16/19
16/19
Contesta la pregunta número cuatro.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
17/19
17/19
Si tienes tiempo, prueba otra sustancia. Escribe los resultados.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
18/19
18/19
Cuando tengas que limpiar, usa una toalla de papel para absorber
el líquido sobre toda tu hoja de mezclas.
el líquido sobre toda tu hoja de mezclas.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
Paso
19/19
19/19
Conversemos:

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
reacción química
1 de 10
un proceso mediante el que una o varias sustancias se mezclan para formar una sustancia nueva

ácido
2 de 10
una sustancia con sabor agrio, por ejemplo, el vinagre o el jugo de naranja

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
disolver
3 de 10
cuando una sustancia se mezcla con otra y parece que desaparece, por ejemplo, lo que sucede cuando agregamos azúcar al té

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
líquido
4 de 10
un estado de la materia, por ejemplo el agua cuando se puede verter

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
gas
5 de 10
un estado de la materia, por ejemplo, el vapor

Please wait…
This video is having trouble loading. You may have lost your Internet connection.
Step 1: Click to Reload this page
Step 2: Click to
Try our other video player
Step 3: Contact your teacher if trouble persists.
Or,
dismiss this message.
mezclar
6 de 10
combinar dos o más cosas
experimento
7 de 10
una prueba que se usa para descubrir más información sobre una pregunta

observar
8 de 10
ponerle mucha atención a algo
sustancia
9 de 10
un material con propiedades específicas

propiedad
10 de 10
algo que puedes observar acerca de un objeto o un material