











DISCUSS (1 of 3): Can you think of any tests you could do, that would help you figure out which idea is true?
Here’s an idea we had...
What if we used a scale? See next slide...
DISCUSS (2 of 3):
Suppose we give you a scale, a tool that measures weight. Using a scale, is there a test you could do to figure out which idea is true?
DISCUSS (3 of 3):
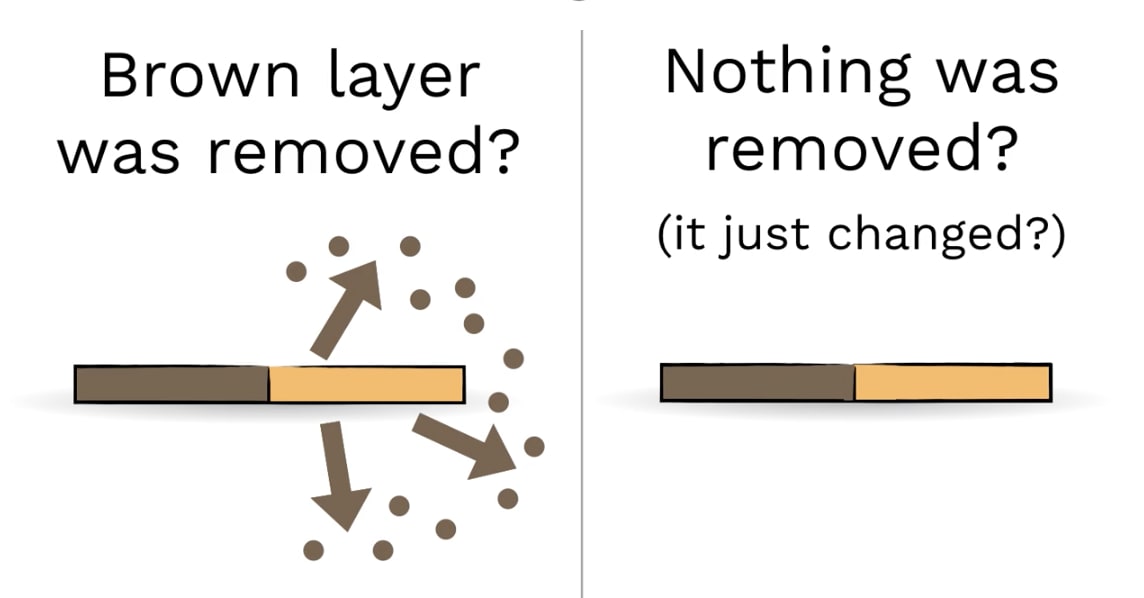
If the vinegar and salt REMOVED the dull copper, then what should we find out when we weigh the penny before and after?





DISCUSS:
Why do you think the alchemist left, never to be heard from again? Was there something he didn’t want the king to figure out?









DISCUSS (1 of 2):
Why do you think we couldn’t see little bits of copper in the liquid?












Grade 5
Chemical Reactions & Properties Of Matter
Dissolving & Particulate Nature of Matter
5-PS1-1, 5-PS1-2
| Alchemist’s Potion, Part 2 worksheet | 30 copies |
|
Alchemist's Potion worksheet from Chemical Magic Lesson 1
Completed in the previous lesson.
|
Details
30 printouts
|
|
Clean-up Supplies (Eg. Paper Towels)
In case of spills.
|
Details
1 roll
|
|
Salt & Vinegar Solution from Chemical Magic Lesson 1
Filled with pennies and a steel nail.
|
Details
1 container
|
|
Dixie Cups (3 oz)
|
8 cups |
|
Medium Binder Clips (1 1/4")
Used to clip Ziploc bags to the plastic bin.
Clothespins or masking tape can also work.
|
Details
8 clips
|
|
Paper Plates
Plastic plates will also work.
|
Details
8 plates
|
|
Plastic Bin
Used to store Ziploc bags for students. Bin must be large enough so that all groups of students can clip their bag to the sides of the bin (8 Ziploc bags for a class of 32).
|
Details
1 bin
|
|
Plastic Spoons
|
4 spoons |
|
Sticker Labels (1" x 3")
Masking tape will also work.
|
Details
16 labels
|
|
Ziploc Bags (Sandwich Size)
Ziploc snack size bags also work.
|
Details
8 bags
|
|
Steel Nails
It’s important that you get steel nails (or steel washers)—NOT stainless-steel and NOT galvanized steel.
Jumbo paper clips will also work, but the results are harder to see.
|
Details
8 nails
|
To do this activity, you must have completed the activity in Lesson 1.
We suggest students work in groups of four. Homeschool students can work on their own, but will need help with some steps.
At the end of the last lesson, you put all the pennies in the Salt & Vinegar solution, added a steel nail when students were gone, and then left the solution overnight. To prepare for this activity, check on that solution before class and look at the nail you put in. All or part of the nail should now be covered by a layer of copper.
Take the nail out of the solution, rinse it off, and set it aside to show your students during the last video. Save the penny-filled solution for students to use in this activity.
Students will first need the following materials:

We then recommend you set up three supply stations around the classroom:

If conditions are just right, copper forms on the steel quickly. But in many cases, students may need to leave their experiments for a few hours or even overnight to see results.
To store students' experiments overnight, we suggest taping or clipping the Ziploc bags to the sides of a plastic bin, with the bags hanging over the inside of the bin. This ensures that the nail remains submerged in the liquid. In the unlikely event that the bags leak, the bin will catch the vinegar.
Grade 5
Chemical Reactions & Properties Of Matter
Dissolving & Particulate Nature of Matter
5-PS1-1, 5-PS1-2
Thanks for your feedback! If you have a question or need help, please contact us. Please consider sharing your review:
Sorry the lesson didn’t go well. We read every single review in an effort to improve our Mysteries.
Thanks for letting us know. We’ll wait to ask you for feedback until after you've actually taught it.
Thanks for the feedback! We read every single review in an effort to improve our Mysteries.
Please follow these steps:
Locked
6:10

Why is the sky blue?
Locked
4:41

Why do we call them doughnuts?
Locked
5:16

Could a turtle live outside its shell?
Your membership is expired. The archive of past Mini Lessons is not included in your limited access.
View pricing